Sample information management system (Biobank)
A set of sample bank resource management system based on the cell sample work flow.
In accordance with operations involved in the work flow of lab samples, to achieve efficient and continuous management of the whole-process recording and all-dimensional quality control of the sample life cycle.
To be applied in hospitals, medical labs, pharmaceutical industry, scientific research units, third-party storage institutions, etc., and can be expanded to industries such as food, chemical, etc.
Safe access
Comprehensive tracking and monitoring of data on sample collection, preparation, quality control and in-storage process, to ensure the security and reliability of the in-storage sample resources.
Open for sharing
Data information is presented to higher authorities for data archiving, and for shared application with the relevant scientific research institutions accessible for cooperation.
Information traceability
Sample-centered, visualized consultation of data information of the whole life cycle of samples, including collection, in-storage and other historical information.
Convenient information retrieval
In scientific research application, complex combinations can be made based on biological resource attributes, genotypes and other information, so as to locate target samples conveniently and quickly.
Compatible applications
Able to build up a whole-process closed-loop management of samples based on different practical application scenes, and to achieve data return of the experimental projects and experimental results after out-of-storage of samples.
High standard
In accordance with GMP standard and the international 21CFRPart11 Digital Signature Standard.

Core Functions
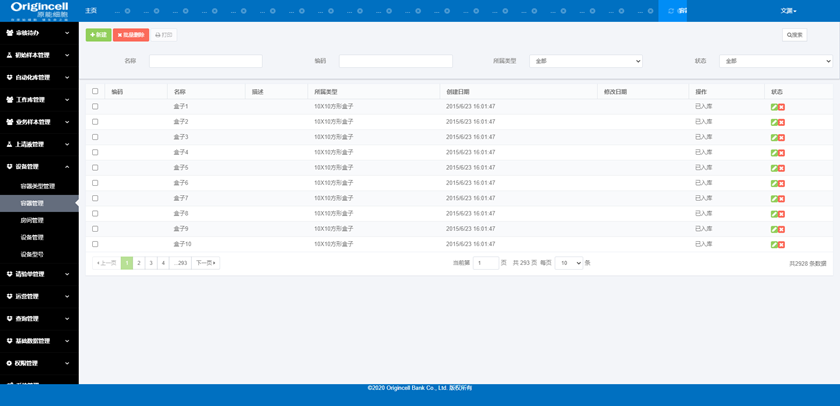
Equipment Management
Management of the information, operation status, capacity, time, type, etc., of equipment, rooms, containers, models and so on
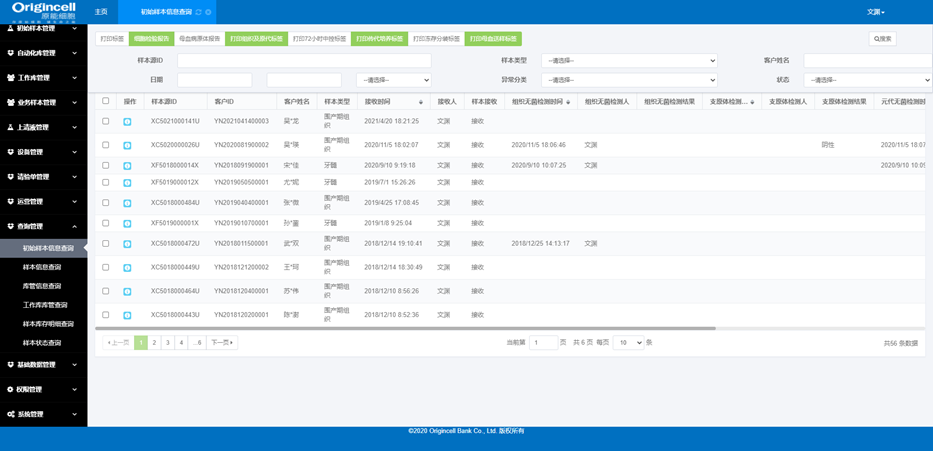
Sample management
Sample receiving, split packaging, in-and-out-of-storage, status, diachronic information, etc.
Quality control
QC upload testing result; QA review; QP review
Permission/menu management
Hierarchical rights management
Third-party data links
Batch import page display of sample data, experiment data, clinical data (HIS, LIS), etc.